
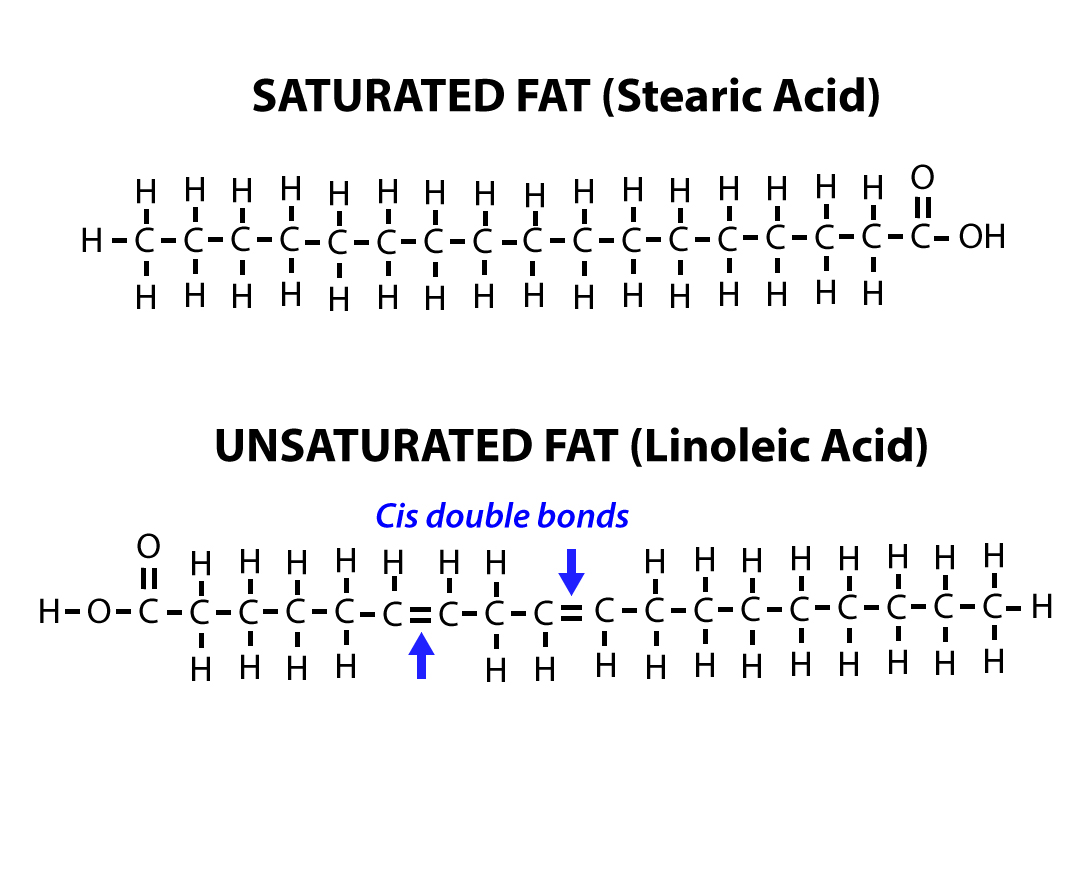
Saturated fats tend to get packed tightly and are solid at room temperature. If there is one double bond in the molecule, then it is known as a monounsaturated fat (e.g., olive oil), and if there is more than one double bond, then it is known as a polyunsaturated fat (e.g., canola oil). Most unsaturated fats are liquid at room temperature and are called oils. When the hydrocarbon chain contains a double bond, the fatty acid is an unsaturated fatty acid.
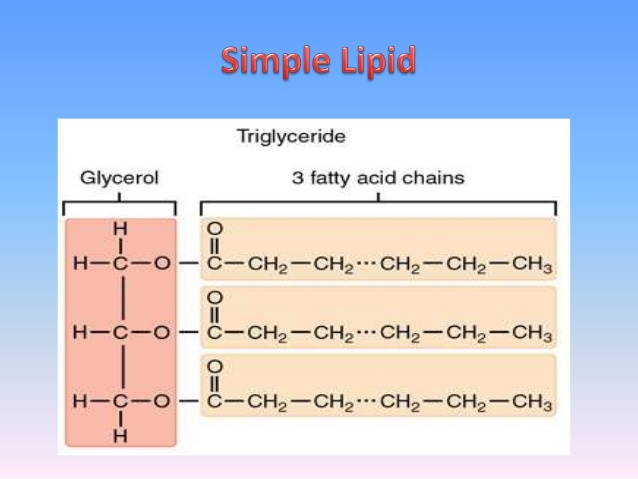
Saturated fatty acids are saturated with hydrogen in other words, the number of hydrogen atoms attached to the carbon skeleton is maximized. In a fatty acid chain, if there are only single bonds between neighboring carbons in the hydrocarbon chain, the fatty acid is saturated. Arachidic acid is derived from Arachis hypogaea, the scientific name for peanuts.įatty acids may be saturated or unsaturated. For example, palmitic acid, a saturated fatty acid, is derived from the palm tree. Some fatty acids have common names that specify their origin. These fats are also called triglycerides because they have three fatty acids. The three fatty acids in the fat may be similar or dissimilar. Lipids include fats, such as triglycerides, which are made up of fatty acids and glycerol, phospholipids, and steroids.ĭuring this covalent bond formation, three water molecules are released. In a fat molecule, a fatty acid is attached to each of the three oxygen atoms in the –OH groups of the glycerol molecule with a covalent bond (Figure 2). Fatty acids have a long chain of hydrocarbons to which an acidic carboxyl group is attached, hence the name “fatty acid.” The number of carbons in the fatty acid may range from 4 to 36 most common are those containing 12–18 carbons. Glycerol is an organic compound with three carbon atoms, five hydrogen atoms, and three hydroxyl (–OH) groups. Lipids include fats, oils, waxes, phospholipids, and steroids.Ī fat molecule, such as a triglyceride, consists of two main components-glycerol and fatty acids. Lipids are also the building blocks of many hormones and are an important constituent of all cellular membranes. For example, they help keep aquatic birds and mammals dry when forming a protective layer over fur or feathers because of their water-repellant hydrophobic nature. Lipids also provide insulation from the environment for plants and animals (Figure 1). Cells store energy for long-term use in the form of fats.
Lipids perform many different functions in a cell.

Non-polar molecules are hydrophobic (“water fearing”), or insoluble in water.

This is because they are hydrocarbons that include mostly nonpolar carbon–carbon or carbon–hydrogen bonds. Lipids include a diverse group of compounds that are largely nonpolar in nature. Hydrophobic lipids in the fur of aquatic mammals, such as this river otter, protect them from the elements. Identify several major functions of lipidsįigure 1.Distinguish between the different kinds of lipids.


 0 kommentar(er)
0 kommentar(er)
